Iron is one of the most useful and versatile metals on planet Earth. This strong, durable metal-shaped civilization goes back thousands of years to ancient times. From the iron tools and weapons of antiquity to the steel skyscrapers and machinery of today, iron built empires and enabled innovations that transformed our existence.
Let’s explore some of the most fascinating fun facts about iron that continue to be essential in the modern world. There are intriguing details about iron’s role in nature, science, technology, medicine, culture, and more. Read on to uncover crazy tidbits and hidden truths about iron that you never knew!
#1 – Iron is the second most abundant metal on Earth.
The first of our fun facts about iron is that it is extremely plentiful, making up about 5% of the Earth’s crust. In fact, iron is the second most abundant metal on Earth after aluminum. The core of our planet is believed to be largely composed of iron. This explains why iron is readily available in various mineral forms and ores across the globe. Iron’s abundance contributes to its indispensability.
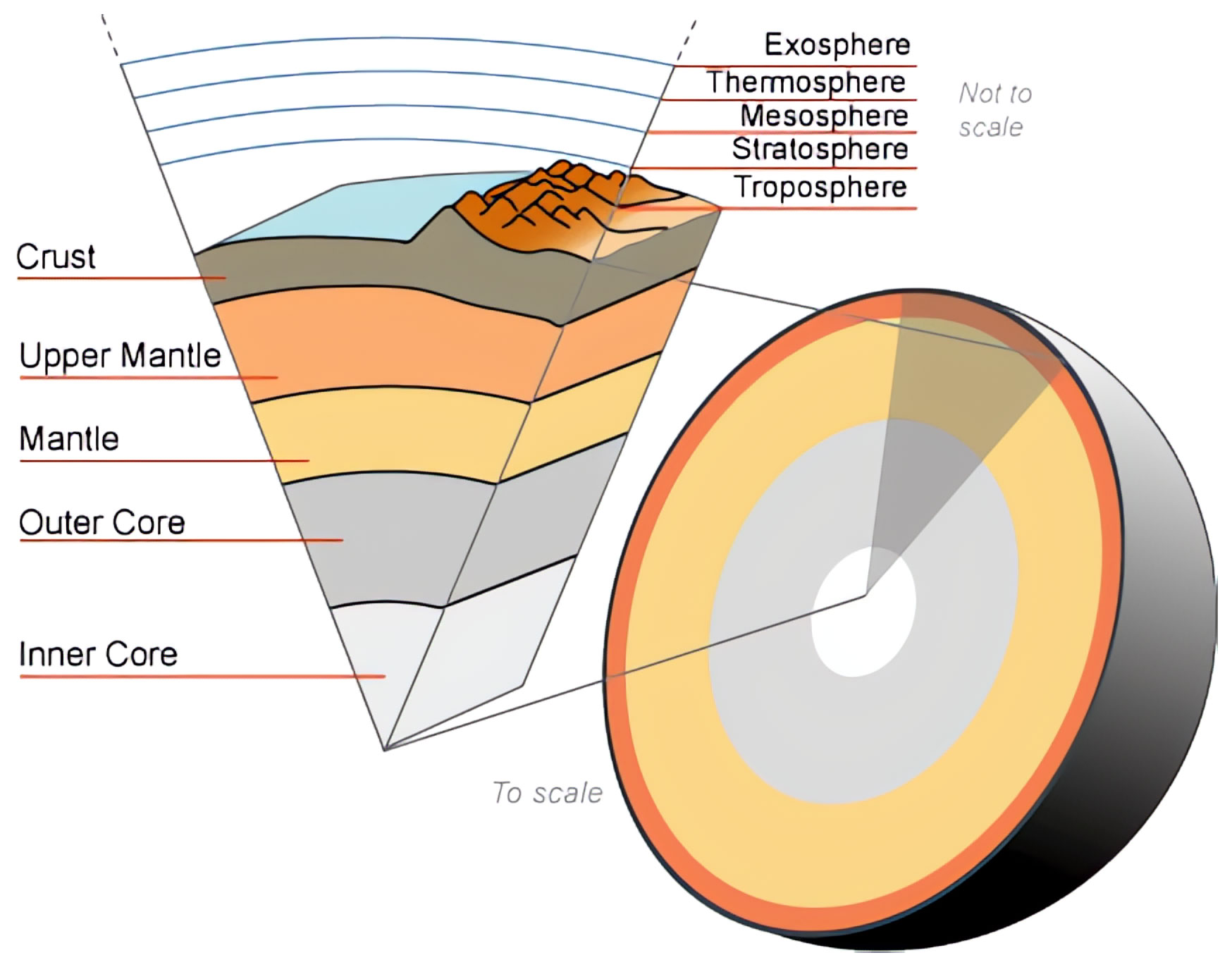
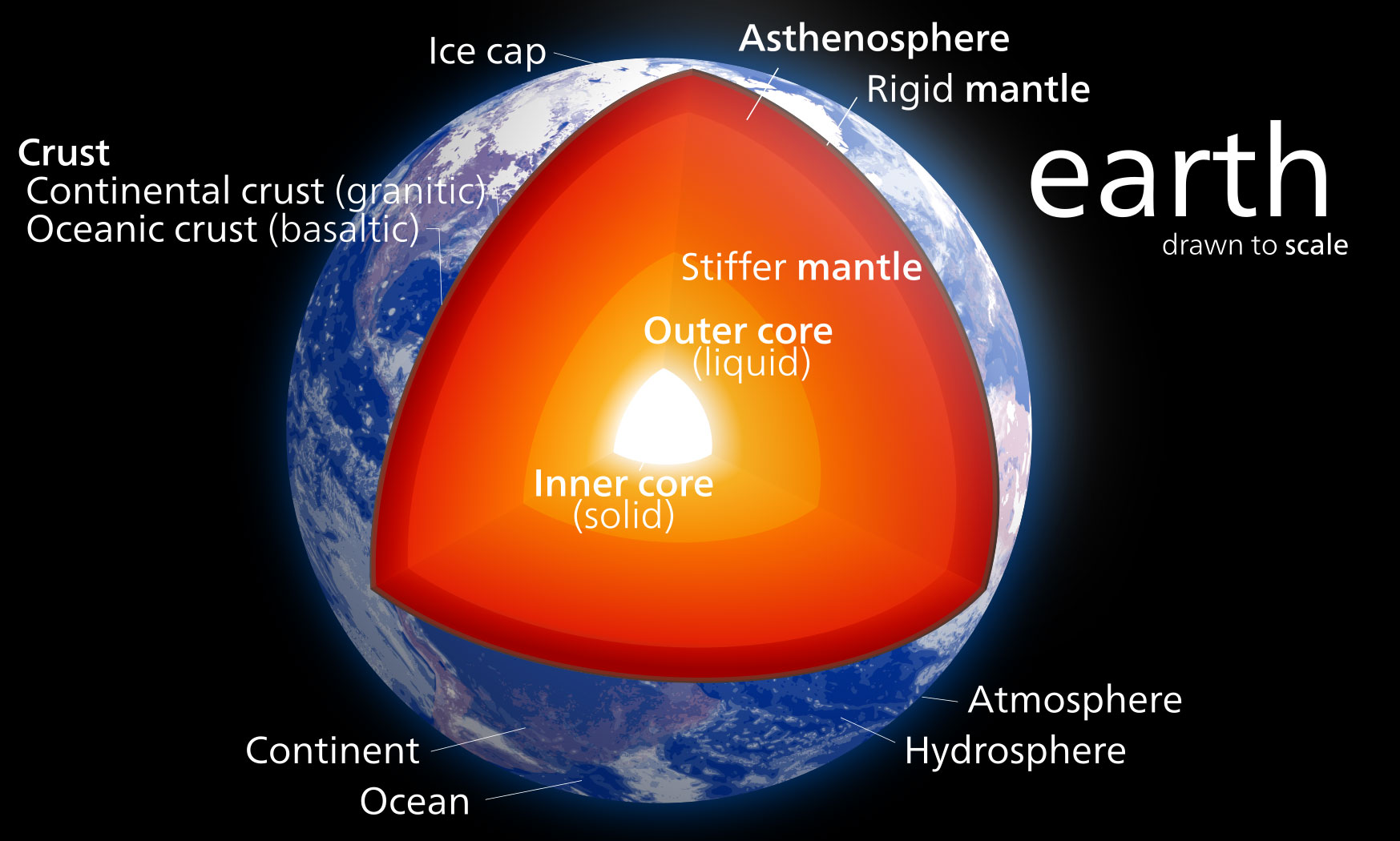
#2 – Iron composes about 80% of the Earth’s inner and outer cores.
Iron makes up about 80% of the Earth’s inner and outer cores by mass. The inner core is solid iron, while the outer core is molten iron. So the middle of our planet is basically a giant iron ball. This strange fact shows how vital iron is to Earth’s formation and existence. The immense pressures in the core give iron unique properties, unlike anything we can produce on the surface.
#3 – Iron is the main component of meteorites.
Another one of the more intriguing fun facts about iron is that it is commonly found in meteorites that originate from asteroids. Nickel-iron alloys are a major component of many meteorites. In fact, iron is the main component overall.
Some meteorites also contain traces of precious metals like gold and platinum. Iron from extraterrestrial objects helped seed our planet with this life-giving metal. It makes you wonder if iron is present throughout the entire universe. Something to think about! If you’d like to learn more about our galaxy, and its planets, check out our fun facts about Mars.

#4 – You cannot make steel without iron.
One of iron’s most important uses is producing steel. You cannot manufacture steel without iron. Steel is an alloy that contains iron and a small percentage of carbon. Sometimes other elements are also added.
The addition of carbon makes steel much stronger and more durable than pure iron. Everything from infrastructure to vehicles to machinery contains steel components. So in an indirect way, iron supports modern civilization. Think of that the next time you drive by a construction site.

#5 – Around 70% of the iron in the human body is found in the hemoglobin of red blood cells.
About 70% of the iron in the human body resides in the hemoglobin of red blood cells. Hemoglobin gives blood its red color and serves to transport oxygen from the lungs to tissues and organs.
Iron is an integral part of the hemoglobin molecule. Iron deficiency can lead to anemia, fatigue, and other problems. This makes iron essential for human life and health. Plants also require iron to form chlorophyll used in photosynthesis. So iron facilitates biological processes in both animals and plants. Handy! Learn more about your body in our facts page about the muscular system.

#6 – Iron is not always magnetic.
One of the more scientifically fascinating facts about iron is that its magnetic properties depend on its crystal structure. The most stable and common form of iron is called alpha iron. Alpha iron happens to be ferromagnetic, meaning it is strongly attracted to magnets. However, when iron is heated above 770°C, it changes into a form called gamma iron. Gamma iron is paramagnetic, meaning it is only weakly attracted to magnets. So iron’s magnetism literally changes with temperature!

#7 – Iron has four allotropes.
The different forms that elemental iron adopts are called allotropes. Iron has four allotropes that depend on temperature and pressure conditions. At atmospheric pressure, alpha iron is stable below 770°C. From 770–947°C, beta iron is stable. Above 947°C up to the melting point of 1538°C, gamma iron is stable. And at extremely high pressures, delta iron emerges. The varying crystal structures give these allotropes different magnetic and other properties.
#8 – Iron has the highest melting point of all the metals in the first transition series.
Another one of our favorite fun facts about iron is that it has the highest melting point of all metals in the first transition series on the periodic table. This includes elements like chromium, manganese, cobalt, nickel, and copper which melt at substantially lower temperatures. Iron melts at 1538°C whereas copper melts at 1085°C.
Its extremely high melting point makes iron well-suited for producing durable tools, machinery, and infrastructure.

#9 – Iron has a bright golden color when it burns in air.
When iron burns in air, it produces impressive bright golden flames with sparks. This happens because iron reacts rapidly with oxygen in the air at high temperatures.
Ancient peoples incorporated iron pyrite into primitive firearms to generate sparks. And flint striking steel creates sparks that can ignite fires. So iron’s flashy tendency to burn brightly shows up in early technologies. Burning iron leaves behind an oxidized mineral called hematite which has a striking blood-red color. Iron is truly one of the most amazing and useful elements.

#10 – The element symbol for iron, Fe, comes from the Latin word for iron, “ferrum.”
In the periodic table, iron’s element symbol Fe comes from the Latin word for iron, “ferrum.” Many of iron’s chemical symbols stem from this root word. For example, Fе2O3 is the chemical formula for iron oxide, which contains one iron atom for every two oxygen atoms. The name “iron” itself originates from earlier words like “iren” in Anglo-Saxon and “iarn” in Old Norse languages that described the metal.
#11 – Iron was known to ancient civilizations as early as 5000 BC.
One of the more intriguing iron facts is that this metal dates back over 5000 years, yet maintains cutting-edge uses today. Iron artifacts first appeared around 3000 BC in Mesopotamia. But iron was not common until the Iron Age beginning in 1200 BC. Ancient peoples produced wrought iron and steel alloys. Today, nanostructures of carbon and iron produce revolutionary materials 1000 times stronger than regular steel. So iron has both ancient roots and futuristic applications.
#12 – Iron is one of the elements that can be produced by nuclear fusion in stars.
One of the most astounding fun facts about iron is that it originates from dying stars much more massive than our sun. Nuclear fusion within stars synthesizes lighter elements into heavier ones. But once fusion processes produce iron, the reaction stops. Iron nuclei have the highest binding energy per nucleon of all elements, so fusing iron actually consumes energy. This kills the star, causing a supernova explosion that scatters iron into space. The iron in our blood came from an ancient cataclysm light years away!
#13 – Iron is one of the most recycled materials in the world.
Almost 90% of refined metal produced today is iron, predominantly to make steel. Steel is unique because it is 100% recyclable without losing strength or durability. Hence steel products are constantly being recycled into new steel. This makes iron one of the most recycled materials on Earth. All the iron mined over history still circulates in reused steel. Recycling transforms iron into a renewable resource.

#14 – Iron can form compounds with a variety of oxidation states.
One neat fact about iron is it can form stable compounds with a wide range of oxidation states, from -4 to +6. Oxidation occurs when an atom loses electrons, while reduction gains electrons. The most common iron compounds are the +2 (ferrous) and +3 (ferric) oxidation states. But complexes like potassium ferricyanide (K3[Fe(CN)6]) with iron in the +3 state have distinctive properties. This compound is insoluble and has a vivid blue color used in blueprints and pigments.
#15 – Iron can be very toxic if ingested in large amounts.
Too much of a good thing can be dangerous. Iron is essential at proper levels, but excess iron is toxic. The human body tightly regulates iron absorption and storage. However, consuming extremely high doses of iron can cause organ damage.
Iron overdose is rare but potentially fatal if left untreated. Excess iron catalyzes free radical reactions which break down lipids, proteins, DNA, and other cellular components leading to cell death. Luckily iron toxicity is not a problem for most people with normal iron levels.
#16 – Iron has been used for many cultural and artistic purposes throughout history.
Finally, iron has deeply influenced human culture and technology over the centuries. For example, the Eiffel Tower framework required over 18,000 tons of iron. Iconic structures like the Statue of Liberty employ an inner iron skeleton. Iron Man’s high-tech suit fuels the imagination. Blood’s iron content connects it symbolically with bravery and sacrifice. Iron’s unique properties continue to shape civilization today as they did thousands of years ago.

Fun facts about iron – Wrap up.
Iron. This ubiquitous substance is integral to infrastructure, industry, living systems, Earth itself, and beyond. Iron built history while fueling innovations that lead into the future. No other element has influenced humanity’s existence to the extent that iron has over millennia.